2011CFDA《进口非特殊用途化妆品备案凭证》
时间:2022-02-22 浏览:1,395
导读:
2011CFDA《进口非特殊用途化妆品备案凭证》
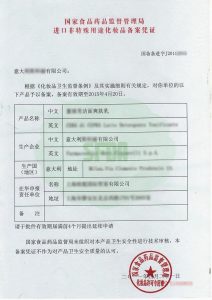
2011CFDA《进口非特殊用途化妆品备案凭证》
责任编辑:本站小编
相关文章:
- [化妆品]2022国家药品监督管理局特殊化妆品注册证样式(天健华成提供)
- [化妆品]2019《进口非特殊用途化妆品备案凭证》式样
- [化妆品]2018版进口非特殊用途化妆品备案凭证
- [化妆品]国产特殊用途化妆品行政许可批件样本
- [化妆品]自贸区进口非特殊用途化妆品备案电子凭证样本
- [化妆品]国产非特殊化妆品备案凭证样式(纸质)
- [化妆品]原CFDA《特殊用途化妆品行政许可批件》样式
- [化妆品]原卫生部《进口非特殊用途化妆品备案凭证》
- [化妆品]自贸区进口非特殊用途化妆品备案电子凭证样本
相关推荐:
- [常见问题]对于无适用的国家标准、地方标准、行业标准的原料,保健食品申报时如何提供原料的生产工艺、质量标准等相关资料?
- [政策法规]野生动植物类保健食品申报与审评规定(试行)
- [审批动态]2024年12月26日化妆品注册批准证明文件(变更、纠错)送达信息
- [审批动态]2023年09月05日化妆品批准证明文件(变更、纠错)送达信息发布
- [常见问题]非特殊类化妆品备案二十个常见问题解答
- [审批动态]2024年12月17日化妆品注册批准证明文件(变更、纠错)送达信息
- [流程周期]对改变产品名称、保质期、食用量,缩小适宜人群范围,扩大不适宜人群范围、注意事项以及功能项目的变更申请(进口)流程
- [申报攻略]国产保健食品批文申报注册10步走(天健华成)
- [材料要求]上海浦东新区进口非特化妆品境内责任人风险管理评估内容及检查要点
- [News]Key Points for Technical Review of Health Food issued
English频道
联系我们
-
86-010-84828041/42
400-6167-168
zhuceabc@zhuceabc.com
咨询微信:
13601366497(化妆品类)
1801335159(特殊食品类)
最新更新
- Main Responsibilities of the National Medical Products Administration
- NMPA Announcement on Issuance and Implementation of the Measures for the Administration of Cosmetics Labels
- NMPA Announcement on Updating the Catalogue of Raw Materials Banned for Cosmetics
- NMPA Announcement on Issuing the Catalogue of Used Cosmetic Raw Materials (Edition 2021)
- NMPA Announcement on Issuing the Working Procedures for the Administration of Supplementary Test Methods of Cosmetics and the Technical Guidelines for the Study and Drafting of Supplementary Test Methods of Cosmetics
- Announcement on Issuing the Technical Guidelines for Submitting Registration and Notification Dossier of Cosmetics (Interim)
- NMPA Announcement on Issuing the Classification Rules and Classification Catalogue of Cosmetics
- NMPA Announcement on Issuing the Technical Guidance for the Safety Evaluation of Cosmetics (2021 Edition)
热门排行
- Contact Us
- BeijingTianjianhuacheng International Investment Co.,Ltd
- Guideline of application and evaluation of children’s cosmetics
- Cosmetics declare FAQ
- Declarations Registered Raiders of Cosmetics
- Procedure
- NMPA Announcement on Updating the Catalogue of Raw Materials Banned for Cosmetics
- Cooperation cases
- EVENTS
- NMPA Announcement on Issuing the Technical Guidance for the Safety Evaluation of Cosmetics (2021 Edition)











